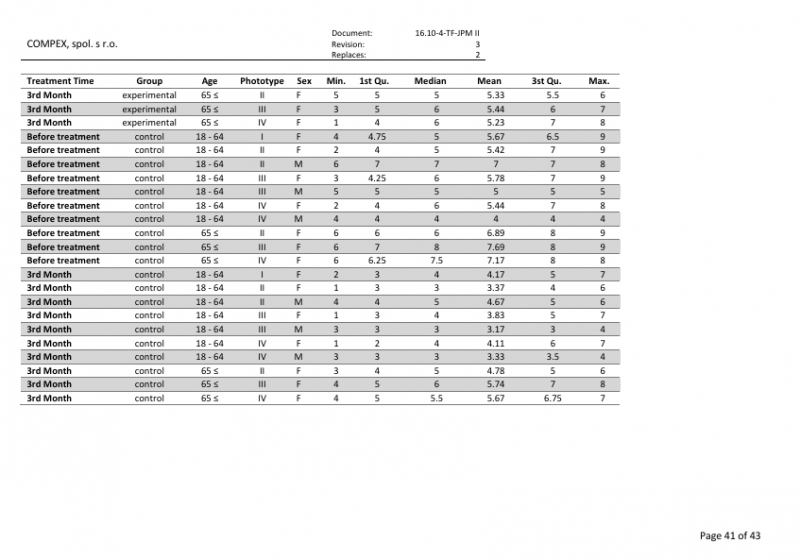
Post-market clinical follow-up study report (PMCF study): Verification of the efficacy and safety of the medical device JETT PLASMA Medical II
Main Results
The primary efficacy endpoint was defined as an improvement of ≥ 1 point on the FWCS from baseline
at the 3-month follow-up after the last treatment. This improvement had to be confirmed by at least
two out of three blinded independent evaluators, based on the subject's photographs. Based on the
primary endpoint, the response rate was calculated and analysed. The results are presented in Table
16.
Table 16 Analysis of the Primary Efficacy Endpoint: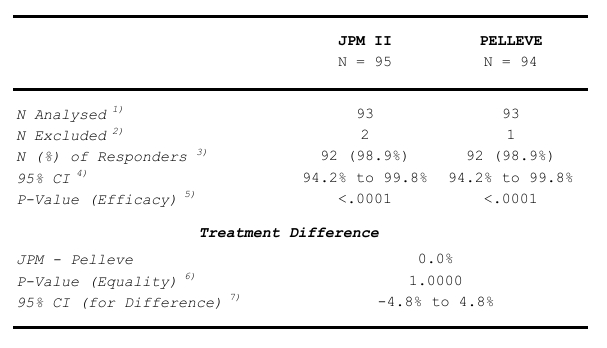
- The number of study subjects in the analysis population.
- The number of study subjects excluded from the analysis due to missing data of the variable.
- The number of responders and the response rate.
- 2-sided 95% confidence interval by treatment group (Wilson).
- H0: Response rate ≤ 0.75 vs. HA: Response rate > 0.75. One-sided.
- An auxiliary test for treatment inequality. It is not intended for the test of non-inferiority.
- 2-sided 95% confidence interval (Newcombe-Wilson) for the treatment difference. This interval is used for the test of non-inferiority.
See full study at: https://drive.google.com/file/d/1y9VhUmFg8EnXpaA_OVhnwP6BCoFJZ3Rf/view?usp=sharing or in the photos attached.